A Sinkhole lot of Chemistry
 Wednesday, June 23, 2010 at 09:28AM
Wednesday, June 23, 2010 at 09:28AM  Sinkhole image by Brian StanberrySinkholes are in. There were reports of a massive one in Guatemala City a few weeks ago and now there are apparently dozens of them in China. Technically the hole in Guatamala City was not really a sinkhole, because it wasn’t made the right way. The “right way” involves a bit of chemistry.
Sinkhole image by Brian StanberrySinkholes are in. There were reports of a massive one in Guatemala City a few weeks ago and now there are apparently dozens of them in China. Technically the hole in Guatamala City was not really a sinkhole, because it wasn’t made the right way. The “right way” involves a bit of chemistry.
Sinkholes are made when sections of bedrock made of carbonate rocks (eg limestone and dolomite) are dissolved. When rainwater or ground water comes in contact with CO2, carbonic acid is formed, which can dissolve sections of bedrock, especially the weaker sections. The Hunan Province does have carbonate bedrock in some areas, but I’m not sure whether these areas are exactly where the sinkholes have been appearing. Once the bedrock becomes fractured, cavities can develop and it can collapse.
The hole in Guatemala City is a case of what geologists call “piping pseudokarst”. This involves the collapse of large cavities that in very weak, loose volcanic material, notably including pumice. In this particular case, the hole was probably caused (or at least exacerbated) by swift running water, brought by tropical storm Agatha.
The process of water coming in contact with CO2 to form carbonic acid is also one of the most worrying impacts of increased levels of CO2 in the atmosphere. CO2 from the atmosphere is absorbed by the ocean, forming carbonic acid. Now consider all the marine fauna who have carbonate structures, such as coral and anything with a shell. That’s probably another story though, but a very important one.
Toothpaste
 Friday, June 18, 2010 at 04:41AM
Friday, June 18, 2010 at 04:41AM If you do what your dentist tells you, you’ll brush your teeth at least twice a day. I do, and – like most people - I use toothpaste. The other day I found myself reading the ingredients and it was quite a list. That got me thinking about the very nature of toothpaste, a substance that – to do its job – must exhibit a wide range of characteristics. So I’ve compiled a list of common toothpaste ingredients and what they do. I also stumbled upon an explanation for why the orange juice you drink after brushing your teeth tastes so foul.
- I use toothpaste. The other day I found myself reading the ingredients and it was quite a list. That got me thinking about the very nature of toothpaste, a substance that – to do its job – must exhibit a wide range of characteristics. So I’ve compiled a list of common toothpaste ingredients and what they do. I also stumbled upon an explanation for why the orange juice you drink after brushing your teeth tastes so foul.
You need to be able to store toothpaste in a small space (eg a tube) but spread it around a larger space (ie your mouth)
Sodium lauryl sulphate (SLS) (AKA Sodium dodecyl sulfate (SDS)) is a surfactant used in a wide variety of products including floor cleaners, shampoos, shaving foams, engine degreasers and bubble baths. In toothpaste it is used primarily as a foaming agent.
Glycerin (AKA glycerol) is a sugar alcohol. It is sweet and viscous so it’s partly there for consistency and partly for flavour.
Carrageenan is any of a family of linear sulphated polysaccharides and is used as a stabilizer, added to prevent constituents separating. Carrageenan is derived from red seaweed (Chondrus crispus) and it is a non-Newtonian fluid, specifically it is thin under shear stress and recovers its viscosity once the stress is removed. This is one reason that toothpaste is easy to squeeze out of the tube, and holds its shape somewhat when outside afterwards, but does not easily drip out of the tube if the lid is left off.
It needs to remove things that are stuck to your teeth
Hydrated silica is a form of silicon dioxide, which has a variable amount of water in the formula. It is also known as silicic acid, a term usually used for its form dissolved in water. It is found in nature, as opal, which has been mined as a gemstone for centuries and in the cell walls of diatoms. It’s used as mild abrasive (this is why toothpaste is sometimes recommended for repairing minor scratches in glass.
Mica refers to silicate minerals (which contain silica, SiO4). They are sometimes called “sheet silicates” because they form a sheet-like crystalline structure. Like
hydrated silica, mica acts as a mild abrasive to aid polishing of the tooth surface. It also adds a glittery shimmer to the paste.
Ideally it should make your teeth stronger and if possible cleaner looking
Sodium fluoride is used to enhance the strength of teeth by the formation of fluoroapatite, a naturally occurring component of tooth enamel. In theory this strengthens teeth to prevent cavities.
Titanium dioxide is used to provide whiteness and opacity.
It should taste nice
Sodium saccharin is the sodium salt version of saccharin (AKA benzoic sulfinide). It is in toothpaste for flavour and is hundreds of times sweeter than sucrose.
See also glycerin, above.
Sorbitol (AKA Glucitol) is another sugar alcohol.
It should discourage the presence and growth of disease-causing organisms
Triclosan (AKA 5-chloro-2-(2,4-dichlorophenoxy)phenol) is a potent wide spectrum antibacterial and antifungal agent.
As for why drinking orange juice after brushing your teeth is so unpleasant, it’s almost certainly has nothing to do with the minty flavour of the toothpaste. Apparently the same unpleasant taste is experienced if you use toothpaste of any flavour (and there are loads of flavours out there), and in other contexts mint and citrus can be a very pleasant combination. It seems that sodium lauryl sulphate might hold the secret, because SLS does more than just help toothpaste lather. SLS also suppresses your sweet receptors, so basically when you drink the orange juice you can taste all the various components of the flavour, except the sweet bits.
Canaries, Chemists and Quantum Mechanics
 Friday, June 18, 2010 at 04:35AM
Friday, June 18, 2010 at 04:35AM I presented a short piece on ‘Einstein-a-go-go’ linking canaries (specifically those in coal mines) to some important advances in chemistry. It goes a little something like this . . .
Most people are familiar with the concept of the “canary in a coal mine”. The phrase refers to the miners’ technique of carrying a canary in a cage to warn of adverse conditions as they moved through a mine. If the canary stopped singing, fell off it’s perch, died or was generally in distress (aside from what you might expect of a small bird placed in a cage and taken underground) this was an indicator that the miners might have come across an area of changed atmospheric conditions. Other animals were also used, but canaries are particularly sensitive to some gases including carbon monoxide and methane – both of which can occur in mines - especially if there has been a fire or explosion.  Image by Alex Clauss
Image by Alex Clauss
If a change in the atmosphere in a mine was detected there were 2 main concerns: that reduced oxygen might lead to asphyxiation or that flammable gasses (such as methane) might cause an explosion, an eventuality made more likely by the use of open flames as a source of illumination. Either eventuality would spoil your day.
Even before miners starting taking canaries into mines efforts were being made to make the whole enterprise safer.
In 1815, Humphry Davy (assisted by Michael Faraday) developed a safety lamp, known as the Davy lamp. The Davy lamp is basically a candle enclosed inside a fine mesh. This mesh screen acts as a flame arrestor. Firedamp (any of the various harmful vapours produced in mines, most commonly methane) can pass through the mesh, but the holes are too fine to allow a flame to propagate through them and cause an explosion. However the flame itself can change in appearance in response to changing gas concentration, alerting the miner to possible danger.
But in 1828 Faraday developed a new version of the Davy lamp (what Bill Gates would have called version 1.2.1). Basically he’d added a movable conical chimney-pot at the top of the lamp, allowing greater control over the mixing of gas and air. Apparently this idea didn't really catch on, at least in mining settings.
Meanwhile, in laboratory settings, a number of chemists had used the idea of the Davy lamp to control the mix of gas and air and thus the specific characteristics of the flame. Previous flame sources, such as furnaces, charcoal burners, spirit lamps and coal gas all had their drawbacks, so scientists were keeping their eyes open for better alternatives. When the University of Heidelberg hired a new professor in 1852, they promised to build him new laboratories. As part of this process the new professor asked the University’s mechanic, Peter Desaga, to design a prototype flame system, based in part of Michael Farraday’s design of more than 25 years earlier.
The design that Desaga produced featured slits for air at the bottom of a cylindrical burner, with the flame igniting at the top. This generated a hot, sootless, non-luminous flame by mixing the gas with air in a controlled way before combustion. His boss, a Professor Robert Bunsen was very satisfied. By the time the building opened early in 1855, Desaga had made fifty of the burners for Bunsen's students. Bunsen published a description two years later, but neither he nor Desaga actually patented it.
Bunsen found that when he fed solutions of metals into the new flame, they emitted spectacular colours. Bunsen’s friend, Gustav Kirchoff, realised that the coloured light could be analysed using a prism. This lead to the development of spectroscopy (and spectroscopes).
Spectroscopy and research on spectral lines by scientists including Niels Bohr (who won a Nobel Prize for his work in this area) lead to greater understanding of the structure of atoms and events on a sub-atomic scale.
This in turn (eventually) led to quantum theory and quantum mechanics, but that’s another story . . .
As it happens canaries were still being used in coal mines as recently as the mid-1980s.
Heads Up
 Saturday, November 21, 2009 at 10:18PM
Saturday, November 21, 2009 at 10:18PM 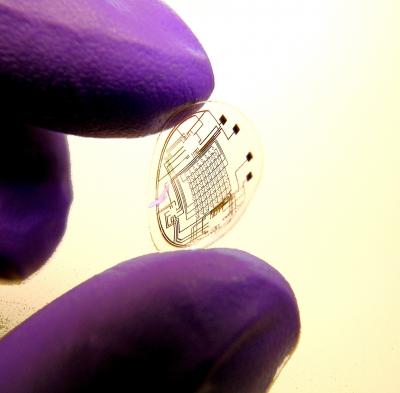 Image: University of WashingtonMany years ago a friend of mine turned up at our house with a new toy. It was basically a combat game and came complete with head-piece, vest and gun for each of 4 players. The gun “fired” some sort of signal – presumably infra-red – which was detected by sensors on the back, front and shoulders of the vest. As the number of shots inflicted upon you increased, you became more “injured” and eventually dead, just like in countless video games.
Image: University of WashingtonMany years ago a friend of mine turned up at our house with a new toy. It was basically a combat game and came complete with head-piece, vest and gun for each of 4 players. The gun “fired” some sort of signal – presumably infra-red – which was detected by sensors on the back, front and shoulders of the vest. As the number of shots inflicted upon you increased, you became more “injured” and eventually dead, just like in countless video games.
We proceeded to run about a local park hiding behind play equipment, hillocks and public toilets and taking shots at each other whenever we could. Other people in the park seemed rather bewildered as we ran, stumbled and giggled our way around them. We were in our twenties and I can only imagine what people thought when they emerged from the public toilet to come face to face with a young man in red goggles holding a gun.
I only remember playing this game once and I don’t think any of us were very good at it, possibly because we were distracted by the cool-ness of the head-up display in the head-piece. It was by this display that each player knew how dead they were becoming and what the team scores were.
Since then (and with added input from various science fiction movies) I have had a special place in my heart (and possibly corneas) for head-up displays. I have been quietly looking forward to the day when mobile phones and perhaps laptops could have their screens replaced by some sort of tiny virtual display. That day is a step closer with the announcement by Babak Parviz and his team at the University of Washington of a new contact lens embedded/imprinted with visual display circuitry. The idea is to produce images that appear to float between 50 cm and 1 metre in front of the user.
They have managed to develop flexible, biologically safe contact lens with an imprinted electronic circuit and tiny LEDs. The circuitry was built using microfabrication self-assembly techniques in which a mixture of various particles is dusted onto a sheet of flexible plastic. Shape-complementarity causes these particles to aggregate into desired structures – in this case the various components of the circuit.
Currently the challenge of powering the lens is met by an antenna that picks up power beamed from a nearby radio source, but the plan is to use from the user's mobile phone.
At present the display is very simple, but as a proof pf principle it’s pretty exciting. Such displays could be used to display speed and performance information for drivers and pilots, to provide information for the visually impaired and of course for gaming. Then there’s web surfing, email, texting, over-laid car navigation systems, sub-titles at the opera . . .
Sasquatch Watch
 Tuesday, October 6, 2009 at 07:56AM
Tuesday, October 6, 2009 at 07:56AM There are some people who really, really want Sasquatch to be real. I suspect they’re not even that fussy – Image by Modulate Sasquatch would be great, but Big Foot, Yeti, Yowie, Yeren, Barmanou, Orang Mawas or Tjutjuna would be fine. Unfortunately there is no credible scientific evidence that any of these creatures exists, although there is fossil evidence for giant primates many thousands of years ago in what is now China, India and Vietnam. Assigned to the genus Gigantopithecus, these primates would have weighed half a tonne and stood up to 3 metres tall (if they stood upright at all, which is in dispute). Anyway, those determined to uncover new evidence might find some hope in recent research reported in the Journal of Biogeography.
Image by Modulate Sasquatch would be great, but Big Foot, Yeti, Yowie, Yeren, Barmanou, Orang Mawas or Tjutjuna would be fine. Unfortunately there is no credible scientific evidence that any of these creatures exists, although there is fossil evidence for giant primates many thousands of years ago in what is now China, India and Vietnam. Assigned to the genus Gigantopithecus, these primates would have weighed half a tonne and stood up to 3 metres tall (if they stood upright at all, which is in dispute). Anyway, those determined to uncover new evidence might find some hope in recent research reported in the Journal of Biogeography.
There have been plenty of reported Sasquatch sightings, especially around Oregon, Washington and North West California. Jeff Lozier, Peter Aniello and Mike Hickerson took geographic data from reported Sasquatch sightings, putative auditory detections, and footprint measurements gathered over the last 65 years. They then used this information to develop an ecological niche model (ENM) too predict the likely distribution of North American Sasquatch, using several software packages and lots of lovely biostatistics.
They take this further to predict possible changes to this distribution that are likely to result from climate change. This might seem like a lot of effort to go to for an animal the existence of which is yet to be established (even Peter Hickerson says that he “doesn’t believe in Big Foot” although he thinks a search is worthwhile) but wait – there’s more. The researchers then went on to show how their methods could be used to develop an ENM for a better-known species, the American Black Bear (Ursus americanus).
As it turns out the predicted range of Black Bears and Sasquatch overlap quite considerably and the researchers conclude that many reported sightings of Big Foot are in fact sightings of Black Bear. Woops.
The article combines very sound scientific methods with a light-hearted approach to expose the risks of over-valuing observation data. Advances in computing power and data storage and manipulation technology have allowed a greater selection of data to be used in ENMs. Generally this is good thing – for example ecologists and conservation biologists need to be able to predict the range of rare and endangered species, with limited first hand data, but the authors are worried that some studies over-value the results of ecological niche modelling.
If any of this is disappointing to Big Foot devotees, don’t be depressed - bears have pretty big feet too . . .
